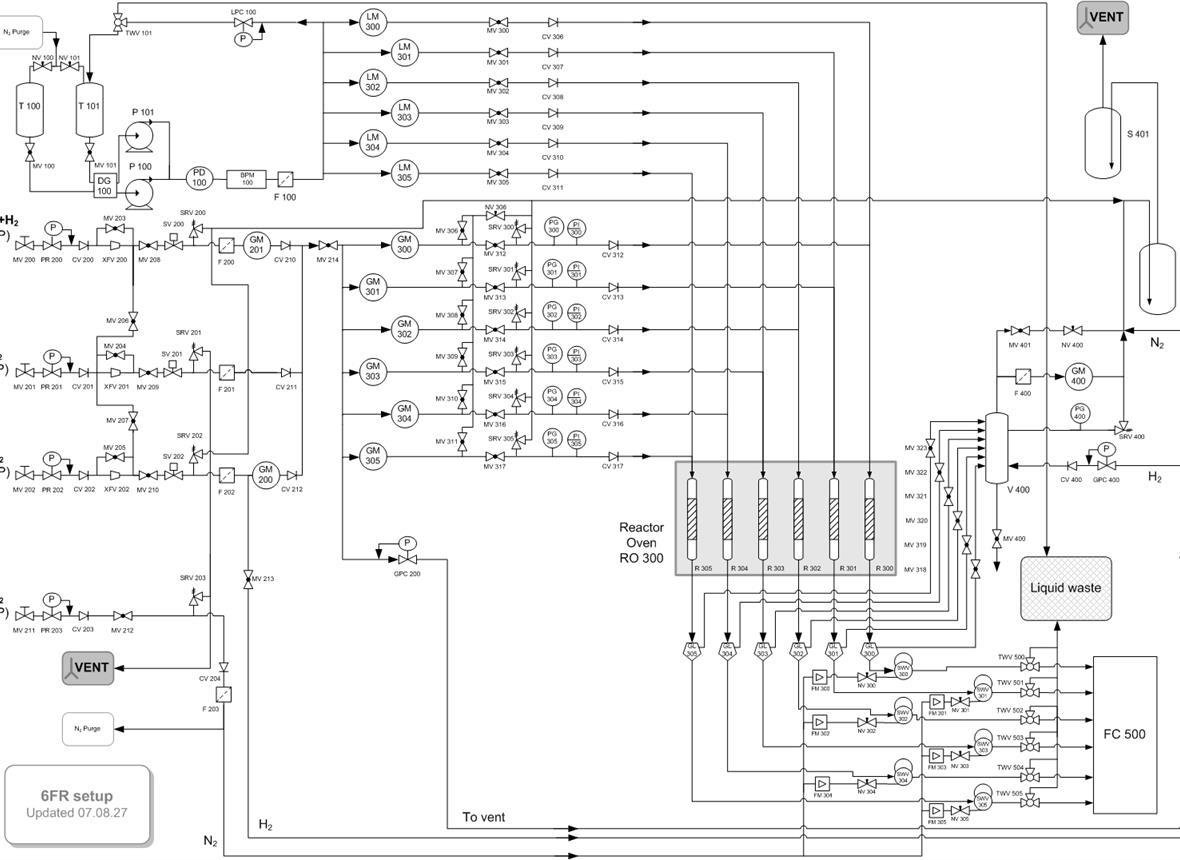
This work explores the synergies during combined reactions of
n-pentane (
nC5) with oxygenates (methanol or dimethyl ether, OX). The experimental runs have been carried out in a packed bed reactor at 500 °C, using a high silica HZSM-5 zeolite-based catalyst with different oxygenate-to-
n-pentane (OX/
nC5) ratios in the feed. A significant enhancement of the
n-pentane conversion occurs for low OX/
nC5ratios in the feed (0.1−0.25), especially when using dimethyl ether (DME). In addition, the presence of
n-pentane reduces the rate of
catalyst deactivation by coking during the conversion of oxygenates. These results have been explained on the grounds of a mechanistic interaction between the reactants: (1) the fast formation of methoxy and
olefin intermediates from oxygenates, particularly from DME, could explain the promotion of
n-pentane cracking, by facilitating the activation of the alkane by hydrogen transfer reactions; (2) the attenuation of deactivation during the conversion of oxygenates could be related to a lower extent of the arene cycle in the dual-cycle mechanism (limiting the polymethylbenzene formation). The analyses of used catalysts by means of temperature-programmed oxidation and confocal fluorescence microscopy have pointed out the higher reactivity of DME than that of methanol also for yielding coke structures.