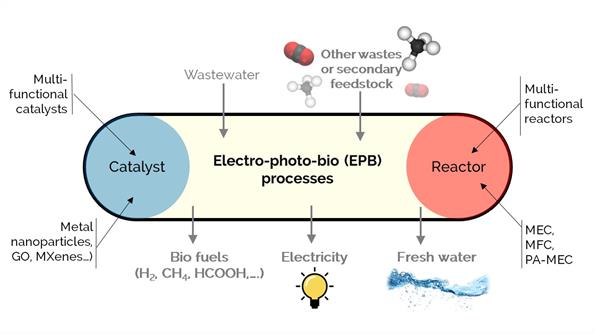
Our long-term commitment to sustainability and a circular carbon economy involves unconventional catalytic conversion processes. We study various processes assisted by electrons, photons, or microorganisms to produce biofuels, chemicals, electricity, or treated water. For example, bio-electro-chemical systems, including microbial fuel cells (MFCs), electrolysis cells (MECs), and photo-assisted cells (PA-MECs), are promising technologies to simultaneously produce renewable energy and clean wastewater using active microorganisms as biocatalysts.
Our work aims to synthesize multi-functional catalysts and reactors to enhance electrical conductivity, photo-efficiency, microbiological affinity, porosity, hydrophilicity, and surface area for carbonaceous electrodes. We work with materials such as graphene oxide, metallic nanoparticles, nitride and carbide basic materials, and MXenes.
We consider new platform technologies to produce renewable biofuel and chemicals and treat wastewater using the nanotechnology and reaction engineering approach as an innovative combination to increase the productivity of these processes.