
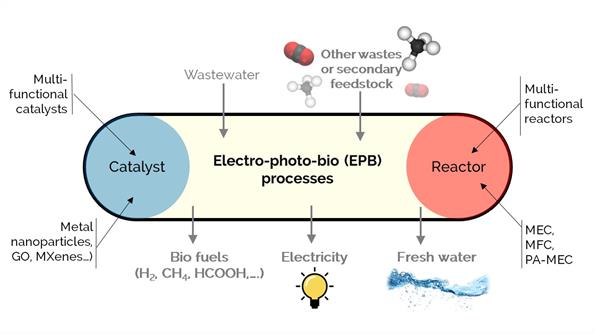
Our long-term commitment to sustainability and a circular carbon economy involves unconventional catalytic conversion processes. We study various processes assisted by electrons, photons, or microorganisms to produce biofuels, chemicals, electricity, or treated water. For example, bio-electro-chemical systems, including microbial fuel cells (MFCs), electrolysis cells (MECs), and photo-assisted cells (PA-MECs), are promising technologies to simultaneously produce renewable energy and clean wastewater using active microorganisms as biocatalysts.
Our work aims to synthesize multi-functional catalysts and reactors to enhance electrical conductivity, photo-efficiency, microbiological affinity, porosity, hydrophilicity, and surface area for carbonaceous electrodes. We work with materials such as graphene oxide, metallic nanoparticles, nitride and carbide basic materials, and MXenes.
We consider new platform technologies to produce renewable biofuel and chemicals and treat wastewater using the nanotechnology and reaction engineering approach as an innovative combination to increase the productivity of these processes.
In this study, we examine the electrochemical-driven reduction of CO2 to methanol at Cu2O/ZnO gas diffusion electrodes in soluble pyridine-based electrolytes at different concentrations. The process is evaluated first by cyclic voltammetric analyses and then, for the continuous reduction of CO2 in a filter-press electrochemical cell. The results showed that the use of pyridine-based soluble co-catalysts lowered the overpotential for the electrochemical reduction of CO2, enhancing also reaction performance (i.e. reaction rate and Faradaic efficiency). Reaction outcome is discussed on the basis of the role that N-ligands play on the mechanism and the inductive effect caused by the electron-releasing or electron-withdrawing substituents of the aromatic ring.
In particular, the maximum methanol formation rate and Faradaic efficiency reached at the 2-methylpyridine (with electron-releasing substituents)-based system with a pH of 7.6 and an applied current density of j = 1 mA cm−2 were r = 2.91 μmol m−2 s−1 and FE = 16.86%, respectively. These values significantly enhance those obtained in the absence of any molecular catalyst (r = 0.21 μmol m−2 s−1 and FE = 1.2%). The performance was further enhanced when lowering the electrolyte pH by adding HCl (r = 4.42 μmol m−2 s−1 and FE = 25.6% at pH = 5), although the system showed deactivation in the long run (5 h) which appears largely to be due to a change in product selectivity of the reaction (i.e. formation of ethylene).