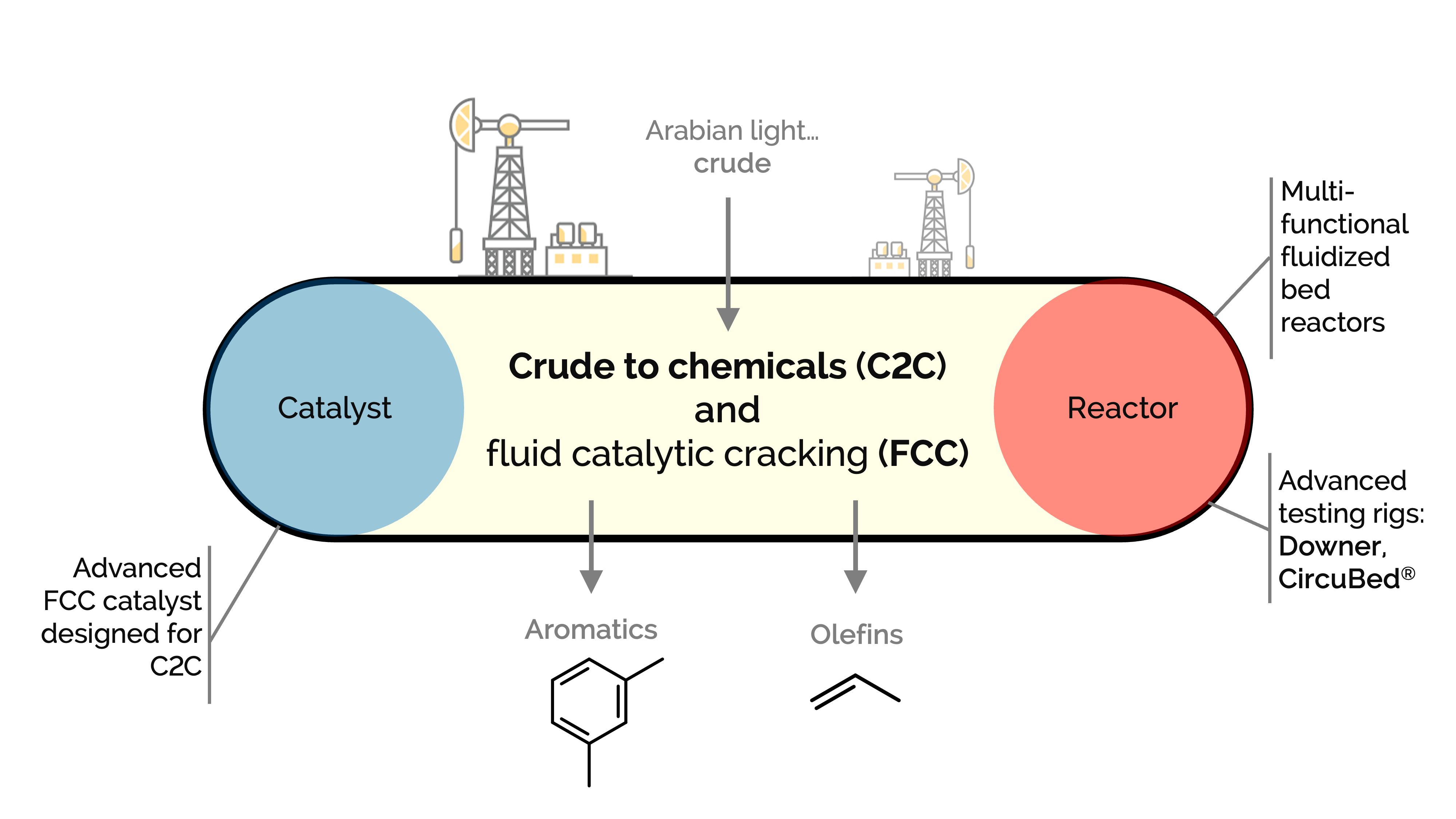
Direct catalytic cracking of crude oil to chemicals could soon dominate the petrochemical industry, with lower fuel consumption and increased production of light olefins and aromatics. We aim to simplify the refinery into a single-step conversion scheme to produce the most demanded petrochemicals.
Using a bottom-up holistic approach, we design a catalytic crude-to-chemicals process toward this goal. We investigate advanced reactors with intrinsic kinetic data and controlled hydrodynamics to improve the process. We study nonlinear multiscale phenomena by coupling hydrodynamics, heat transfer, and reaction kinetics.
We use particle image velocimetry and optical probes, kinetic modeling, computational particle-fluid dynamics, and optimization approaches to improve operating scenarios and develop innovative reactor prototypes.
We focus on the catalyst, reactor, and process levels to enhance and intensify the system. We are optimizing several state-of-the-art laboratory- and pilot-scale units, including a CircuBed®, a downer, and a multifunctional fluidized bed reactor.