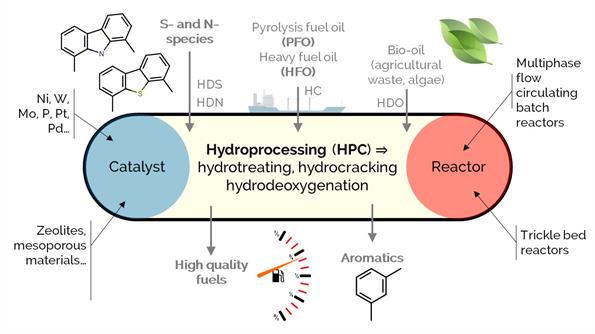
Hydroprocessing is a well-implemented and versatile refinery conversion strategy, comprising a wide array of reaction routes such as: (i) hydrotreating, aiming for the hydrogenation of unsaturated hydrocarbons and the removal (hydrogenolysis) of heteroatoms such as sulfur or nitrogen; (ii) hydrocracking, for promoting C–C bond scission and the partial saturation of aromatics; or (iii) hydrodeoxygenation, for the specific removal of oxygen moieties. In this project, we investigate the conversion of highly polyaromatic feedstock like heavy fuel oil (HFO), pyrolysis fuel oil (PFO), or bio-oils from different biomass sources (i.e., agricultural waste, algae) for quality improvement and obtaining products with higher added value.
We seek new (thermo-) catalytic strategies and improved heterogeneous catalysts with increased activity and stability. We put advanced analytical characterization techniques (i.e., nuclear magnetic resonance, high-res mass spectrometry) to work and combine their results with modeling and statistical tools.