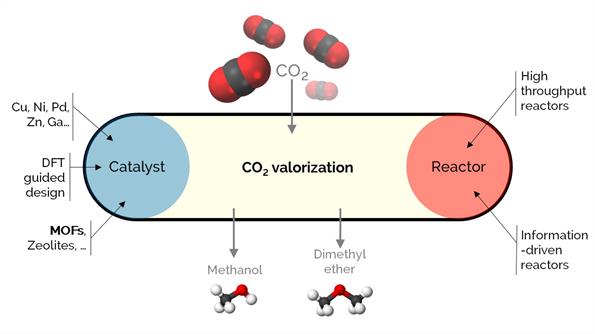
Unarguably, CO₂ is a crucial concern affecting climate change. To cope with or solve the issue, viable valorization strategies are required to efficiently use CO₂, allowing for a circular economy. We aim to convert CO₂ into CO, methane, methanol, dimethyl ether, or E-fuels.
Our activities in CO2 conversion are related to (i) analyzing the stability of industrially relevant catalysts under realistic conditions and (ii) developing new catalytic materials based on Cu. In (i), we are developing reactors that augment the kinetic information: (a) in situ and operando spectroscopic reactors that work under (close to) working conditions to study structure-performance relationships, (b) periodic reactors with transient or variable conditions over time or space. In (ii), we work mainly with novel materials such as metal-organic frameworks (MOFs).
We guide the design of these catalysts based on stability and using density functional theory (DFT) and microkinetic modeling.