The increasing world energy demand accelerates the depletion of fossil fuels, which consequently boosts the research and development of alternative and viable energy sources. Ammonia (NH3) is a dense, carbon-free energy and hydrogen vector. It can provide on-site hydrogen via catalytic decomposition or cracking.
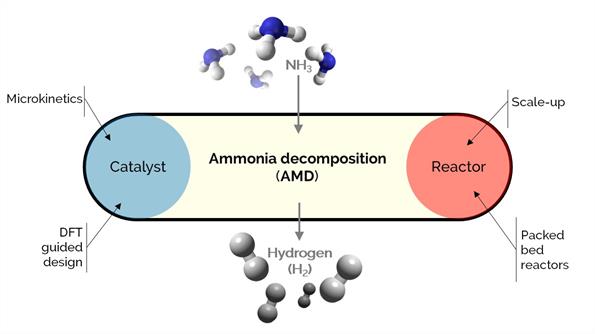
Our work covers the fundamentals of the microkinetics (using benchmark catalysts such as Ru-based and cheaper, novel alternatives such as Co-Ba-Ce-based) to the reactor modeling.
We use DFT-guided and microkinetic modeling to help understand the rates and catalyst performance. Whereas the reactor modeling from the laboratory scale to the industrial-scale mandates considering the heat-mass transfer effects for efficient implementation of the process.