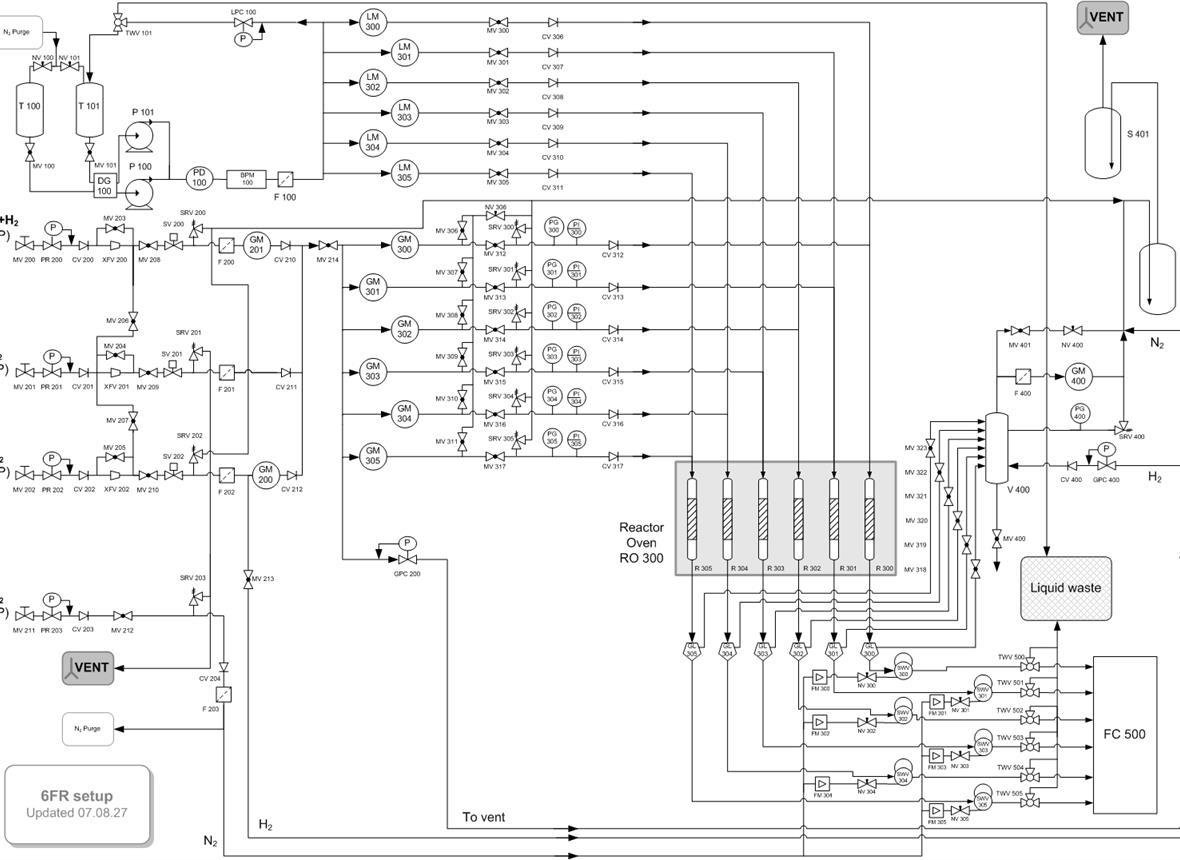
A kinetic model is proposed in order to quantify product distribution in the ring-opening (using high hydrogen concentration in the reaction medium) of methylcyclohexane (MCH) over a catalyst based on HZSM-5
zeolite. The model is based on a reaction scheme proposed by Cerqueira et al. for methylcyclohexane cracking at atmospheric pressure, which has been modified in order to include the effect of hydrogen over the individual reaction steps. The experimental results used for estimating the kinetic constant were obtained in a fixed bed isothermal reactor in a wide range of conditions, i.e. 250–450 °C; WHSV = 0.5–10.5 h−1 (
τ = 0.095–2 gcat h gMCH−1); pressure = 5–80 bar; H2/MCH molar flow ratio = 4–79; conversion = 0–100%. The kinetic model proposed can be regarded as a basis for the proposal of models for ring-opening reactions of more complex naphthenic feedstock from a prior hydrogenation step involving aromatic refinery streams of secondary interest.